Abstract
Introduction: Allogeneic hematopoietic cell transplantation (alloHCT) is the only potentially curative treatment available for acute myelogenous leukemia (AML). It is a medically complicated and resource intensive procedure for which there are patient-, provider- and system-related factors that may impact its utilization. While Medicare coverage for alloHCT may help address a major financial barrier to access, there is still an unmet need due to other factors. This study intended to examine trends and factors associated with utilization of alloHCT and to estimate unmet need for alloHCT among Medicare beneficiaries with AML.
Methods: This retrospective cohort study included all patients with a diagnosis of AML identified in the Medicare claims data from 2010 through 2016. Primary patient selection criteria included: primary or secondary diagnosis of AML, age limit (65-74), and continuous enrollment (for at least 180 days after AML diagnosis in Part A and Part B fee-for-service programs).
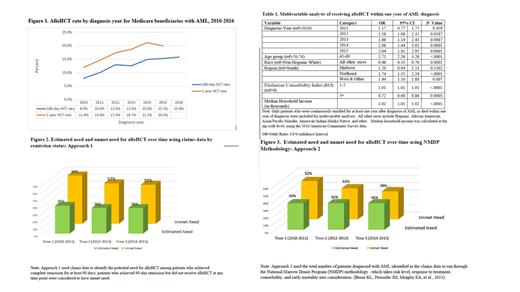
To study trends in utilization, the transplant rates were calculated as the number of patients who received an alloHCT within 180 days and one year of diagnosis (numerator) divided by the total number of patients diagnosed with AML within each diagnosis year (denominator). Transplant rates within one year of diagnosis were further adjusted by patient characteristics, including age group, sex, race, residential region, and Elixhauser Comorbidity Index (ECI). A multivariable logistic regression was utilized to identify factors associated with the likelihood of receiving alloHCT within one year of diagnosis.
Two approaches were applied to estimate unmet need for alloHCT. The first approach (Approach 1) used claims data to identify the potential need for alloHCT among patients who achieved complete remission for at least 90 days; patients who achieved 90-day remission but did not receive alloHCT at any time point were considered to have unmet need.
In the second approach (Approach 2), the total number of patients diagnosed with AML in the claims data was run through the National Marrow Donor Program (NMDP) methodology , which takes estimates of risk level, response to treatment, comorbidity, and early mortality into consideration. Overall estimated need and unmet need from 2010-2015 and over different time periods were evaluated for both approaches.
Results: Among the 5,974 patients diagnosed with AML from March 1, 2010 through June 30 th, 2016, 1226 patients (21%) received an alloHCT by the end of 2016. Trend analysis results suggest that utilization of alloHCT increased from 2010 to 2016 (P < 0.0001) (Figure 1). The likelihood of receiving alloHCT within one year of diagnosis was found to be associated with diagnosis year, age, race, geographic region, ECI, and population-level median household income (Table 1).
Both approaches estimated that approximately 36% of the diagnosed patients were in need of alloHCT between 2010 and 2015. The overall unmet need was estimated as 59% and 43% based on the claims data approach and the NMDP methodology, respectively. Despite the differences in estimated unmet need between the two approaches, the unmet need for alloHCT was found to trend down over time (Figures 2 & 3).
Discussion and Conclusions:
Medicare coverage facilitates access to the health care system and receipt of health services when a need for treatment or care is recognized. Utilization of alloHCT has increased over time among Medicare beneficiaries with AML. However, there are persistent differences in utilization of alloHCT by age, race, geographic region, comorbidity, and socioeconomic status, indicating disparities in access to alloHCT among this population. The results of estimated need and unmet need for alloHCT may be affected by the lack of cytogenetics and molecular information in administrative claims. To minimize this limitation, we used NMDP methodology for validation and found a similar estimation of potential need for alloHCT and downward trend in unmet need. Although the unmet need for alloHCT for AML has improved over time, policy efforts, research, and continued education are needed to close the gap between the actual utilization of alloHCT and unmet need for this potentially curative treatment.
Saber: Govt. COI: Other.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal